
| Type: | Intermediates/Pharmaceutical Intermediates |
| Product name: | Triphenylphosphine |
| CAS NO: | 603-35-0 |
| Molecular weight: | 262.29 |
| EC NO: | 210-036-0 |
| Molecular formula: | C18H15P |
| InChI: | InChI=1/C18H15P.BrH/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15- 18;/h1-15H;1H |
| Product description: | Triphenylphosphine, the basic raw material of rhodium phosphine complex catalyst, has wide uses in domestic petrochemical industry. Triphenylphosphine is also used in the pharmaceutical industry, organic synthesis, analysis and other fields. Triphenylphosphine can also be used as a brightener for dye technology, polymer polymerization, an antioxidant for color film development, a stabilizer for polyepoxidation, and an analytical reagent. Triphenylphosphine, also known as triphenylphosphine, triphenyl (base) phosphine, Triphenylphosphine, phosphine triphenyl, triphenol phenyl ester, Triphenylphosphine, etc., is also called triarylphosphine in the old literature. Product name: t Abbreviation: PPh3 Molecular formula: C 18 H 15 P (is a triphenyl substitution of phosphine.) Molecular weight: 262.30 CAS number: 603-35-0 EINECS number: 210-036-0 Molecular configuration: triangular Cone Property Appearance: White loose powder. Solubility: easily soluble in alcohol, benzene and chloroform; slightly soluble in ester; almost insoluble in water. Density (relative to water): 1.194 (25°C) Melting point: 80.5°C. Boiling point 377°C (91kPa). Flash point (open cup) 180 ℃. Uses: Triphenylphosphine is the basic raw material of rhodium phosphine complex catalyst, and has broad Uses in domestic petrochemical industry. Triphenylphosphine is also used in the pharmaceutical industry, organic synthesis, analysis and other fields. Triphenylphosphine can also be used as a brightener for dye technology, polymer polymerization, an antioxidant for color film development, a stabilizer for polyepoxidation, and an analytical reagent. 1. It is used as raw material for organic synthesis, polymerization initiator, antibiotic drug clindamycin, and standard sample for the determination of phosphorus by organic trace analysis. 2. Preparation of palladium, iridium, rhodium, nickel and other complex catalysts, Wfttig reagent, triphenylphosphine dihalide deoxygenation (N-pyridine oxide, nitrosobenzene, hydroperoxide), desulfurization, debromination reagent. A-bromonitro compounds are made to nitriles. React with aliphatic diazo compounds to synthesize α-ketoaldehyde and β-ketoacid ester. Beckmann rearrangement. Dequaternization of pyridine bell salts. It is used in some syntheses with bromine iodine, tetrachloro(bromo) carbon, N-brominated butadiene imine, etc. 3. Triphenylphosphine is a fairly commonly used reducing agent, and in most cases the reaction is driven by the formation of triphenylphosphine oxide (a thermodynamically favorable reaction). In addition, Triphenylphosphine is widely used as a ligand for metal catalysts. Storage method: Storage precautions: Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Package is sealed. It should be stored separately from oxidants and edible chemicals, and should not be stored together. Equipped with the appropriate variety and quantity of fire equipment. Storage areas should be provided with suitable materials to contain spills. |
| Alias: | triphenylphosphine; triphenylphosphine; triphenylphosphine; triphenylphosphine |
| Structural formula: | 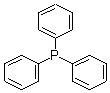 |